If you’ve had recurrent postprandial bloating, chronic diarrhea or constipation, iron‑deficiency anemia, persistent fatigue, migratory arthralgia, or a pruritic vesicular rash, you might be reacting to gluten. Symptoms can be gastrointestinal, neurocognitive, dermatologic, or systemic, and they often vary in timing and severity. You’ll want to know which patterns predict celiac disease, non‑celiac gluten sensitivity, or another cause.
Digestive Symptoms and Bloating
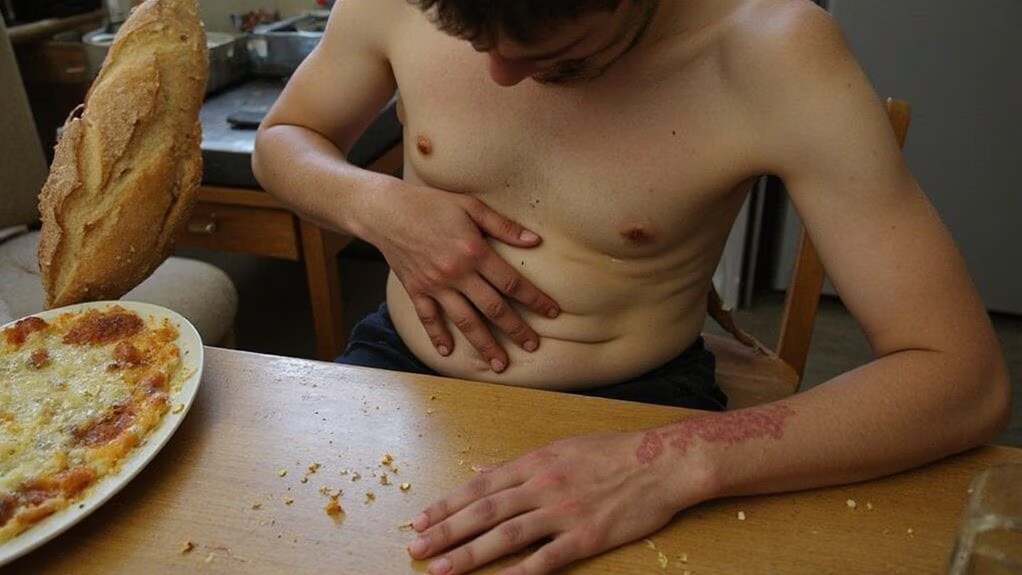
Frequently, you’ll present with postprandial bloating and altered bowel habits when gluten triggers intestinal dysfunction. You experience abdominal distension, visible luminal gas accumulation, and fluctuating stool frequency consistent with functional and organic enteropathy. Objective assessment should include timed symptom diaries, validated symptom scales, breath hydrogen testing for small intestinal bacterial overgrowth, and serologic markers for celiac disease and non-celiac gluten sensitivity. Endoscopic biopsy remains the diagnostic gold standard when villous atrophy is suspected. You’ll monitor response to a controlled gluten-elimination trial with blinded re-challenge to ascertain causality. Management integrates targeted dietary algorithms, microbiome-directed adjuncts, and iterative biomarker evaluation to optimize symptom remission and minimize unnecessary restriction while supporting translational research and personalized therapeutic innovation. You should collaborate with clinicians experienced in precision gastrointestinal medicine.
Headaches and Migraine Patterns
You should monitor the timing of headaches relative to gluten exposure, since delayed-onset presentations occurring hours to days after ingestion are described in gluten-related disorders. Use systematic symptom diaries and time-stamped food logs to quantify trigger timing patterns, latency, frequency, and severity. This temporal profiling helps you distinguish immediate hypersensitivity from delayed neuroinflammatory mechanisms and guides targeted diagnostic testing and elimination trials.
Delayed-Onset Headaches
Often delayed-onset headaches emerge 24–72 hours after gluten exposure and can mimic migraine or tension-type patterns, with unilateral throbbing, photophobia, phonophobia, or diffuse pressure reported in cohorts with non-celiac gluten sensitivity and celiac disease. You should consider immune-mediated mechanisms: gluten-driven cytokine release, anti-gliadin antibodies, and complement activation that may provoke neuroinflammation. You’ll evaluate biomarkers (transglutaminase, anti-gliadin IgG/IgA), neuroimaging to exclude structural causes, and standardized headache scales to quantify phenotype. You must integrate dietary exposure history with objective testing to increase diagnostic specificity. Therapeutically, controlled gluten elimination trials and anti-inflammatory strategies have yielded symptomatic improvement in observational studies. Emerging research targets gut-brain axis modulation and blood–brain barrier integrity as mechanistic and therapeutic domains. You should document response metrics and iterate treatment based on objective change.
Trigger Timing Patterns
Typically, headache onset after gluten exposure follows discrete temporal patterns that help you distinguish immediate immune or histaminergic reactions (within minutes to a few hours) from delayed neuroinflammatory responses (commonly 24–72 hours). You should track onset latency, symptom evolution, and co-occurring autonomic signs to phenotype attacks; immediate headaches often present with urticaria, flushing, rhinorrhea and rapid cortisol/IgE responses, whereas delayed migraines correlate with cytokine cascades, microglial activation, and blood–brain barrier perturbation. Use prospective diaries and timestamped dietary challenges with controlled gluten dosing to establish causality. Analyze temporal congruence with biomarkers (CRP, cytokines, serum tryptase) and neuroimaging when feasible. Integrate machine-learning clustering to refine patient subtypes and tailor intervention timing, since timing predicts therapeutic targets and monitoring windows. You’ll prioritize rapid-response protocols for clinical trials.
Skin Rashes and Eczema-Like Irritation
How gluten triggers cutaneous reactions involves an IgA-mediated immune response with granular IgA deposits at the dermal papillae that produce intensely pruritic, papulovesicular or eczematous lesions most classically seen in dermatitis herpetiformis but sometimes presenting as eczema-like irritation. You’ll notice clustered papules, excoriations, and symmetric extensor distribution; direct immunofluorescence confirms granular IgA. Management emphasizes strict gluten exclusion and topical or systemic therapies guided by severity and dapsone responsiveness. Diagnostic workflow:
- Clinical pattern recognition
- Serology for tissue transglutaminase
- Dermatopathology with immunofluorescence
- Therapeutic trial and objective scoring
Consider point-of-care immunofluorescence and microbiome-modulating adjuncts in refractory cases, and monitor skin response quantitatively to correlate with dietary adherence. Integrate wearable tracking, AI-assisted image analysis, and cloud-secure platforms to enhance monitoring, personalize interventions, and accelerate translational research globally efficiently.
Persistent Fatigue and Brain Fog
Because gluten-related disorders produce systemic inflammation, malabsorption, and immune-mediated neural injury, patients may present with persistent fatigue and cognitive slowing (“brain fog”) that impairs daily function. You may experience sustained low energy, diminished processing speed, impaired working memory, and reduced executive function that don’t correlate with sleep duration. Objective testing can reveal attenuated attention and slowed psychomotor response; biomarkers often show nutrient deficiencies (iron, B12, folate), elevated inflammatory cytokines, or antibodies (tTG, EMA) depending on phenotype. Clinical management integrates diagnostic serology, targeted nutrient repletion, and a monitored gluten-elimination trial; prospective cognitive measures and wearable activity metrics support response assessment. For innovators, combining neurocognitive testing, metabolomics, and personalized dietary algorithms refines diagnosis and optimizes rehabilitation trajectories. You’ll require longitudinal follow-up and data-driven treatment adjustments regularly.
Joint Pain and Muscle Aches
You may experience inflammatory joint pain characterized by migratory arthralgia and synovitis temporally related to gluten exposure. You can also develop proximal muscle weakness with reduced endurance and systemic fatigue consistent with gluten-related myopathy. You should be evaluated for autoimmune arthritides (e.g., rheumatoid arthritis), as studies show increased co-occurrence suggesting shared immunopathogenic mechanisms.
Inflammatory Joint Pain
Although classically confined to the gastrointestinal tract, gluten-related disorders can produce inflammatory joint pain and myalgias via systemic immune activation. You may experience symmetric arthralgia, inflammatory synovitis, or episodic oligoarthritis that responds variably to gluten removal. Diagnostic strategy should include serology for celiac-specific antibodies, HLA typing when indicated, CRP/ESR monitoring, and targeted imaging to document synovial inflammation. Consider the following clinical markers:
- Distribution: small joint symmetric involvement versus large joint oligoarthritis.
- Temporal pattern: postprandial flares correlating with gluten exposure.
- Biomarkers: anti-tTG, anti-deamidated gliadin peptides, elevated CRP/ESR.
- Imaging: ultrasound or MRI evidence of synovitis or tenosynovitis.
You should coordinate care with gastroenterology and rheumatology for precision management. Early recognition and a gluten-free trial with objective outcome measures can inform mechanistic treatment decisions and patient-centered strategies.
Muscle Weakness and Fatigue
When present, muscle weakness and fatigue in gluten-related disorders reflect a spectrum of processes—immune-mediated myopathy, small-fiber neuropathy, metabolic and micronutrient deficiencies (iron, B12, vitamin D), or disuse—often overlapping with systemic inflammation and arthralgia. You should assess pattern: proximal vs distal weakness, fatigability, and sensory symptoms. Obtain targeted testing: CK, inflammatory markers, B12, ferritin, 25(OH)D, thyroid panel, and celiac serologies; consider EMG, nerve conduction studies, and skin biopsy for small-fiber pathology. Muscle biopsy may demonstrate inflammatory infiltrates or atrophy in select cases. Therapeutic strategy integrates strict gluten exclusion, correction of deficiencies, and graded physiotherapy; immunomodulatory therapy is reserved for biopsy-proven immune myopathy. You’ll monitor objective strength metrics and biomarkers to gauge response and innovate personalized rehabilitation protocols. Adjust interventions based on quantified functional outcomes periodically.
Autoimmune Arthritis Link
Because gluten-related disorders alter mucosal immunity and increase intestinal permeability, they can trigger systemic autoimmunity that often manifests as inflammatory arthritis and diffuse myalgias. You should pursue targeted evaluation: serology (tTG, EMA), HLA typing, and rheumatologic assessment when joint symptoms accompany GI signs. Synovitis often shows lymphocytic infiltration and elevated CRP, and may partially respond to gluten exclusion. Monitor objective measures (DAS28, MRI) and antibody titers. Novel mechanisms implicate transglutaminase neoantigens and molecular mimicry. Management integrates disease-modifying therapy with strict gluten-free diet and iterative reassessment. Consider researching interventions aimed at gut barrier repair and antigen-specific tolerance. You should prioritize objective endpoints in trials and practice routinely.
- Serology and genetics
- Imaging and objective scoring
- Diet plus pharmacotherapy
- Translational trials and biomarkers
Mood Swings, Anxiety, and Depression
How might gluten-related pathology contribute to mood dysregulation? You should consider immune-mediated neuroinflammation: in celiac disease and non-celiac gluten sensitivity, translocation of gliadin fragments and zonulin-mediated intestinal permeability can trigger systemic cytokine release (IL-6, TNF-α) that crosses the blood–brain barrier, altering neurotransmitter metabolism. You’ll observe correlations between anti-neuronal antibodies and cognitive-affective symptoms; mechanistic studies suggest microglial activation and reduced serotonin precursors. Clinically, patients report anxiety, labile mood, and depressive syndromes that sometimes remit with strict gluten exclusion, supporting causality in subsets. Biomarkers (serology, cytokine profiles, intestinal permeability assays) combined with controlled elimination-challenge protocols can refine diagnosis and guide personalized interventions, advancing translational models for neuroimmune–gut axis modulation. You should integrate longitudinal monitoring and data-driven algorithms to optimize therapeutic decisions and predict outcomes in real-time.
Unexplained Weight Loss or Gain
If you note unexplained weight loss or gain alongside gastrointestinal or systemic symptoms, consider gluten-related pathology as a potential contributor. You should evaluate temporal correlation between gluten exposure and weight changes, document dietary intake, and assess for malabsorption or altered appetite regulation mediated by enteric inflammation. Diagnostic strategy integrates serology, genetic risk markers, and targeted imaging or endoscopy when indicated. Management trials of gluten exclusion can be diagnostic and therapeutic but require structured monitoring. Key considerations include:
- Temporal association: onset relative to gluten introduction or re-exposure.
- Objective measurement: serial weight and body composition.
- Inflammatory activity: stool markers, CRP, fecal calprotectin when relevant.
- Therapeutic trial: supervised gluten-free intervention with outcome metrics.
You should coordinate care with specialists and use validated protocols.
Nutrient Deficiencies and Anemia
If you have gluten intolerance—particularly celiac disease—mucosal damage to the proximal small intestine impairs iron absorption and commonly produces iron-deficiency anemia. You can also develop vitamin B12 and folate deficiencies from more extensive small‑bowel involvement, which contribute to macrocytosis, neuropathy, and impaired hematopoiesis. You should assess CBC, ferritin, transferrin saturation, serum B12 and red‑cell folate, and initiate targeted supplementation while treating intestinal mucosal recovery with a strict gluten‑free diet.
Iron-Deficiency Anemia
Because gluten-mediated enteropathy commonly targets the proximal small intestine, you should suspect iron-deficiency anemia in patients with gluten intolerance; malabsorption of dietary iron at the duodenum and proximal jejunum produces a characteristic microcytic, hypochromic anemia with low serum ferritin and transferrin saturation. You’ll evaluate hemoglobin, MCV, ferritin, transferrin saturation, and CRP to distinguish absolute deficiency from inflammation-driven sequestration. Endoscopic biopsy confirming villous atrophy strengthens causal link. Treat by instituting a strict gluten-free diet and targeted iron replacement, monitoring response with ferritin and hemoglobin at 8–12 weeks post-treatment. Consider the following diagnostic and management priorities:
- CBC with indices and reticulocyte count
- Serum ferritin and transferrin saturation
- Duodenal histology for mucosal assessment
- Oral or IV iron selection based on severity and absorption
B12 and Folate Deficiencies
Although gluten-mediated enteropathy primarily injures the proximal small bowel, it can impair folate absorption directly and contribute to vitamin B12 deficiency through altered diet, small-bowel bacterial overgrowth, or concomitant gastric/pancreatic dysfunction, so you should maintain a low threshold for testing both vitamins in patients with anemia. Evaluate serum folate, methylmalonic acid, and homocysteine to distinguish deficiencies; measure intrinsic factor antibodies, gastrin if B12 deficiency is present. You should consider duodenal biopsy or serology for celiac disease when deficiencies are unexplained. Treat confirmed folate or B12 deficiency promptly with replacement and address malabsorption via gluten-free diet, antibiotics for documented bacterial overgrowth, or pancreatic enzyme/gastric acid restoration as indicated. Monitor hematologic response and neurologic signs and clinically integrate point-of-care diagnostics and metabolic profiling to optimize outcomes.
Oral Signs: Mouth Sores and Dental Issues
When you have gluten-sensitive enteropathy, oral mucosal lesions and dental abnormalities frequently serve as extraintestinal markers of disease activity and past malnutrition. You may present with recurrent aphthous stomatitis, enamel hypoplasia, delayed eruption, or mucosal atrophy; these findings reflect immune-mediated mucosal injury and historical nutrient deficits. Document lesion morphology, distribution, and timing relative to gluten exposure. Consider interdisciplinary diagnostic pathways integrating dental records, serology, and histopathology to elucidate pathophysiology. Novel diagnostic algorithms and smart imaging can enhance detection sensitivity.
- Recurrent aphthae: painful, shallow ulcers.
- Enamel defects: hypoplasia, pitting, discoloration.
- Salivary changes: xerostomia, altered flow/composition.
- Gingival and mucosal atrophy: friability, erosions.
You should correlate oral findings with nutritional panels and celiac-specific serology to drive precision management strategies and translational research initiatives.
When to Seek Medical Evaluation
If you have persistent diarrhea, steatorrhea, unexplained weight loss, iron‑deficiency anemia, or recurrent oral ulcers, seek medical evaluation promptly, as these are common presentations of gluten‑sensitive enteropathy. You should pursue serologic testing (tTG‑IgA, total IgA) and consider HLA‑DQ2/DQ8 genotyping when diagnosis is uncertain. If serology or symptoms suggest enteropathy, your clinician will recommend duodenal biopsy via upper endoscopy to confirm villous atrophy and intraepithelial lymphocytosis. Prior to testing, don’t start a gluten‑free diet, because it reduces diagnostic sensitivity. Manage concurrent micronutrient deficiencies and bone density with targeted assays (iron studies, B12, folate, 25‑OH vitamin D, DXA). Refer to gastroenterology and dietetics for coordinated care and innovation‑driven monitoring, including noninvasive biomarker research protocols when available. Document outcomes and participate in clinical trials if appropriate now.
Conclusion
You should consider gluten intolerance when you notice recurrent postprandial bloating, chronic diarrhea or constipation, iron‑deficiency anemia, aphthous ulcers, enamel defects, delayed headaches, persistent fatigue or cognitive slowing, migratory arthralgias, or pruritic papulovesicular eruptions. Track temporal correlations with gluten exposure and symptom response to elimination diets, and document objective findings (serology, nutrient levels). Seek medical evaluation for diagnostic testing including celiac serology and duodenal biopsy or supervised reintroduction when clinical suspicion persists, and management guidance.